HPCUS and HPUS Basics
Introduction
In this post you will find information about/ our purposes (as stated in our Articles of Incorporation), our legal status, and our organizational structure and governance.
Articles of Incorporation (1980, in Washington, DC)
The specific and exclusively charitable, educational, and scientific purposes for which the Convention was formed are:
- To accumulate pertinent information and publish and to sell the Homoeopathic Pharmacopoeia of the United States and any additions or supplements thereto,
- To promote the art of healing according to the natural laws of cure from a strictly homoeopathic standpoint,
- To diffuse knowledge among the laity and professionals in the health care field concerning homoeopathic principles through means of publications,
- To research and obtain a thorough knowledge of the pathogenicity of each drug offered for inclusion in the (HPUS) as a homoeopathic drug,
- To develop criteria for eligibility of drugs for inclusion in the (HPUS),
- To serve as a repository for homoeopathic literature and drugs,
- And generally to do, perform, undertake, direct, encourage, and investigate all aspects and functions of any nature directed to the furtherance of homoeopathic healing
The Legal Status of the HPUS as an Official Compendium
May 6, 2022 By Al Lorman, American Association of Homeopathic Pharmacists Counsel (Reprinted with Permission)
Pharmacopoeias have been around for thousands of years. Historically, they provided the medical community with critical information necessary to make (and sometimes use) drugs. In many ways, pharmacopoeias were the first consumer protection vehicle in the world of pharmaceuticals.
The Homeopathic Pharmacopoeia of the United States (in its various iterations) is older than the Federal laws which regulate drugs. The first edition of the HPUS was published in 1897, nine years before the passage of the Pure Food and Drugs Act of 1906. That Act recognized the United States Pharmacopoeia but was silent on the HPUS, an omission that was remedied in the Federal Food, Drug, and Cosmetic Act of 1938 (FD&C Act). Curing that omission was largely the work of Sen Royal S. Copeland (D-NY), a homeopathic physician who was the principal Senate sponsor of the legislation and the former president of the New York City Board of Health. (Sadly, Sen. Copeland died just four days after the Federal Food, Drug and Cosmetic Act was passed and before President Franklin D. Roosevelt signed it into law.)
Since 1938, then, the HPUS has been recognized as an “official compendium.” What exactly does that mean?
First, the FD&C Act defines a drug, in part, as “articles recognized in the . . . official Homoeopathic Pharmacopoeia of the United States . . . or any supplement . . . .” So a substance included in the HPUS is going to be considered a drug by FDA.
Second, the HPUS is an “official compendium.”
Third, a drug is considered to be adulterated “if it purports to be or is represented as a drug the name of which is recognized in an official compendium, and its strength differs from, or its quality or purity falls below, the standard set forth in such compendium. Such determination as to strength, quality, or purity shall be made in accordance with the tests or methods of assay set forth in such compendium . . . .”
Fourth, a drug is considered to be misbranded if the drug is recognized in an official compendium and its label fails to bear “the official title thereof in such compendium . . . .”
You will note that the FD&C Act says nothing about the role of an “official compendium” in regulating uses of a drug. Nor does it require that a drug be recognized in an official compendium.
So, it is possible to take a very narrow view of the role of the HPUS. And, for many years, that narrow view did prevail. But with the revival of interest in homeopathy in the 1970’s, the HPUS began to assume a more prominent role in homeopathy. The issuance in 1988 of Compliance Policy Guide 400.400, Conditions under Which Homeopathic Drugs May Be Marketed (since withdrawn), noted the legal status of the HPUS as an official compendium, as discussed above. The CPG also stated, not very elegantly, that, “Documentation must be provided to support that those products or ingredients which are not recognized officially in the HPUS, an addendum to it, or its supplements are generally recognized as homeopathic products or ingredients.” Thus, from FDA’s perspective in 1988, a drug listed in the HPUS was not only official, but also “generally recognized as homeopathic.” Since the CPG contained no explicit premarket application requirement, the phrase, “documentation must be provided…” appears to have been what was left after FDA initially considered requiring an HPUS monograph to come within the ambit of the CPG.
As homeopathy has grown, the practical role of the HPUS has grown as well. In addition to substantially increasing the data required for acceptance of a new drug (including clinical data), the HPUS has taken the lead in developing guidance for compliance with various cGMP requirements. These efforts have included, for the first time, substantial interactions with FDA scientific personnel. And, unlike the USP, the HPUS continues to monitor the safety and toxicology profile of monographed drugs and make monograph changes when appropriate. Thus, the HPUS has gone well beyond its original statutory role to undertake a variety of actions “directed to the furtherance of homeopathic healing,” in the words of the Homeopathic Pharmacopoeia Convention of the United States.
HPCUS and HPUS — what is the difference?
The HPUS (Homoeopathic Pharmacopoeia of the United States)is the “official compendium,” the listing of homeopathic medicines. It contains drug monographs, which, once listed, become standards that manufacturers must adhere to when formulating homeopathic drug products.
The HPCUS (Homoeopathic Pharmacopoeia Convention of the United States) is the corporation that maintains, develops, and publishes the HPUS.
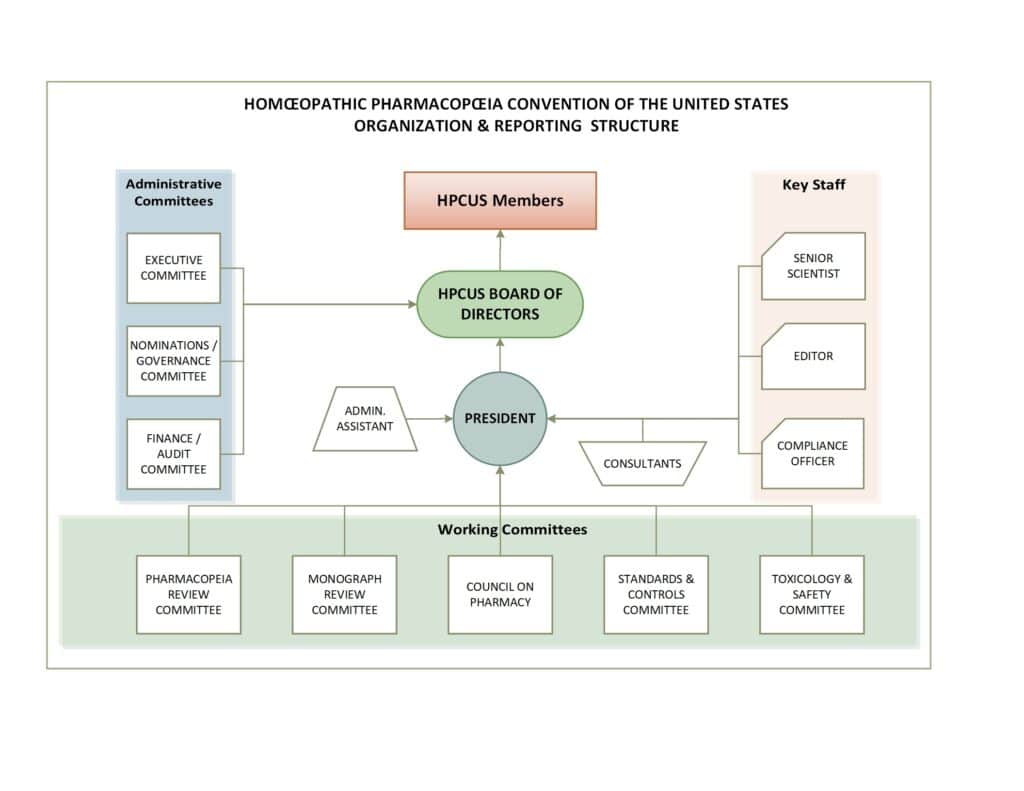
Our Organizational Structure
The HPCUS is a 501(c)3 tax-exempt charitable and educational institution. We are organized into committees for technical and administrative functions. To become a member of the Convention a formal application must be submitted. Being primarily a scientific and technical organization, we look for specific skillsets and expertise that will aid us in publishing the HPUS. The Board of Directors reviews the applications. A unanimous vote of the Board is required for acceptance. A newly accepted member is given “associate member status” for at least one year and assigned to a committee by the President of the Convention. They may advance to “active” membership after demonstrating their value to the convention.
Committees are charged with making recommendations to the Board of Directors, which makes all final decisions in all matters. Committees are strongly urged to operate by consensus, although votes on their decisions are recorded for the record.
The Board of Directors is a self-electing entity. When we wish to add a director, we recruit from the membership. The President and other officers are elected annually. As in the committees, we operate by consensus but record votes as a matter of course.
All members of the Convention comply with our published policies regarding Conflict of Interest, Confidentiality of Information, and others. Those policies are listed in the footer of all pages on this site.
We have an Editor, who coordinates various functions within the Convention and is responsible for maintaining the database of monographs and technical documents, as well as working with sponsors of drug monographs to ensure complete submissions and other matters pertaining to the submission process. The editor is also responsible for many other operations involved in publishing the HPUS.
We have a Senior Scientist who coordinates all the scientific and technical aspects of our work and maintains our ongoing relationships with subscribers to the HPUS and academic and research organizations.
The diagram below summarizes our structure: