Submitting a Monograph
Guidelines for Homeopathic Drug Provings
Approved April 2015
7. Data Analysis
Data compilation and analysis by the Proving team is a key factor in the translation of raw Proving data into a meaningful clinical drug picture that can be used by a prescriber. (24) The data analysis process must be carefully designed to avoid excessive inclusion of non-specific, non-characteristic data, yet ensure that the most characteristic and dependable data for homeopathic prescribing is maintained.
Monograph reviewers are instructed to evaluate Proving outcomes and analysis according to these guidelines (see Chart 2) to ensure that an adequate Proving outcome has been achieved. An analysis process to extract dependable homeopathic prescribing indications from a drug Proving is required to contain the following dimensions (as described in information that follows):
-
- 1st dimension All symptoms occurring during the Proving
- 2nd dimension Proving symptoms with relative characteristic assessment
- 3rd dimension Characteristic symptoms (a highly individualized subset)
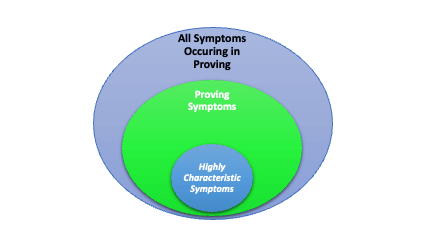
All Symptoms occurring during the Proving should be recorded according to criteria in Section 5. This group will include symptoms that are typical for the prover historically (but not related to the Proving), symptoms unrelated to the Investigational Proving Substance (IPS), symptoms due to placebo effect, and symptoms due to the IPS.
- Proving Symptoms
These are those symptoms or signs that are recorded during the Proving period where causality by the IPS is possible. Symptoms that occur in a severity, duration and frequency consistent with historical tendency (i.e. Unchanged (U) symptoms) of a subject should not be reported as Proving symptoms. Likewise, care should be taken to exclude from this category any symptoms related to a cause that can confidently be determined to be external to the Proving. Proving Symptoms should be defined using qualitative criteria that have been established prior to Proving initiation, and should include:
Criteria to define a significant change in any objective measure, if such measures are used in the Proving.
Qualifiers for subjective (reported by subject) symptoms including:
a) New symptoms, not previously experienced (N)
b) Unexpected a change representing worsening or aggravation of ongoing or recurring symptoms (C-)
c) Unexpected a change representing improvement in ongoing or recurring symptoms (C+)
d) Unexpected recurrence of past symptoms (R)
(These criteria should be the same criteria used in decision-making for non-repetition of the IPS as described in Section 4.8 of these guidelines.)
- Characteristic Features
The following list is provided as a recommended template to use for evaluation of characteristic features of Proving symptoms:
a) New symptoms with marked or unexpected severity, duration or frequency in the subject.
b) Ongoing or recurring symptoms present during the Proving that have been unexpectedly and markedly improved.
c) Ongoing or recurring symptoms that have been unexpectedly and markedly worsened
d) Symptoms that recur from the past but have not occurred in the 12 months preceding the Proving.
e) Symptoms that display alternation with another symptom in a single volunteer in such a way that the alternation is strongly individualizing.
f) Symptoms associated with modalities or concomitant symptoms occurring in other parts of the same prover.
g) Symptoms that involve multiple body parts or organs in a similar manner or multiple symptoms within the same subject with a similar associated modality, forming an easily recognizable pattern of reaction.
h) Similar symptoms occurring in multiple provers. Such symptoms may be related by similar sensation, modality, or body system and can be recognized through a qualitative analysis similar to “Red-Line” symptom reporting in homeopathic literature (1).
- Highly Characteristic Symptoms
These symptoms obtained in the Proving represent those Proving symptoms produced in a subject that are of particular value from a homeopathic perspective, and only include highly individualistic symptoms. Reporting Highly Characteristic Symptoms that occur during a Proving is strongly recommended to help establish an adequate homeopathic clinical picture of the IPS. Highly Characteristic Symptoms are one of the primary criteria used in evaluation of Proving outcomes. When reporting Highly Characteristic Symptoms such symptoms should be reported in a binary fashion (highly characteristic or not). When Highly Characteristic Symptoms are reported from the Proving, symptoms will be evaluated using the following criteria:
Strongly individualizing symptoms must be well described by the prover or observing supervisor.
Proving symptoms will be considered to be Highly Characteristic Symptoms only when they have strongly characteristic and individualizing features. These types of symptoms are often described as being highly peculiar, strange, or rare in their nature.
Highly Characteristic Symptoms are recognizable as those symptoms that are sufficiently individualizing to allow a clinician to reasonably consider the use of the medicine based upon that clinical feature alone.
- Clinical Synopsis of the IPS
Well-designed Provings create an opportunity to develop a coherent remedy picture for the IPS. A remedy picture represents the influence of the IPS upon the complex human biologic system as observed in patterns of reaction in one or more subjects. While all Proving symptoms should be considered in this process, Characteristic Symptoms play a more important role due to their specificity, individuation, and consistency with good homeopathic clinical practice.
The following elements will be considered in this part of the monograph review:
a) Quality and Number of Proving symptoms.
b) Quality and Number of reported characteristic symptoms.
c) Frequency of similar Proving symptoms or observations within a single prover, across multiple subjects in the same Proving, or if appropriate, in other Provings of the same IPS.
Sufficiency of remedy picture development will be evaluated by the Pharmacopeia Revision Committee (PRC). Sufficiency determination will be determined by PRC consensus and Board of Director approval. The Proving results must attain at least one of the following:
a) Presence of 1 or more Highly Characteristic Symptoms
b) A discernible clinical picture emerges in one prover or across multiple provers
c) Presence of 3 or more proving symptoms with sufficient characteristic quality to provide an adequate amount of data for clinical use.
Homeopathic Provings that produce few or no Proving symptoms and few or no characteristic symptoms in subjects receiving the verum IPS may not provide sufficient indications for clinical use of the IPS. In turn, lack of sufficient clinical indications for the IPS may result in a determination of inadequacy of the Proving for the monograph.
Symptoms reported in subjects who received control allocation may be included in the monograph Proving publication provided that such symptoms are clearly noted as placebo-related symptoms.
Pharmacopeia revision committee proving quality review template
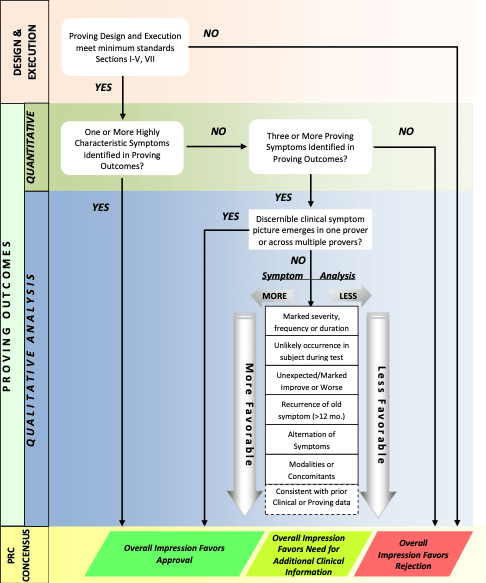
Chart 2
(1) Lippe A Von,Macfarlan D (ED.). Keynotes of the Homoeopathic Materia Medica. Philadelphia: Boericke and Tafel, 1915. Reprinted by B. Jain, Delhi, 1977.
